Research Roadmap
and Treatment Pipeline
Researchers are making tremendous progress on a variety of therapeutic interventions for SCN2A-related disorders, including small molecules (traditional drugs), ASO therapy, gene therapy, and targeted neurological interventions. Explore the research roadmap and treatment pipelines to see where we’re going and how far we’ve come!

Research Roadmap
FamilieSCN2A has worked with a robust network of experts to chart the course to a cure for SCN2A-related disorders. This detailed research roadmap, entitled “A Patient Organization Perspective: Charting the Course to a Cure for SCN2A-Related Disorders,” was published in February, 2024 in Therapeutic Advances in Rare Disease (Vol. 5, 1 - 20) (See article below).
The research roadmap, along with the continuously updated treatment pipeline, illustrate the tremendous growth and momentum toward the Foundation's vision of a world with effective treatments and cures for ALL SCN2A-related disorders.
A PATIENT ORGANIZATION PERSPECTIVE: CHARTING THE COURSE TO A CURE FOR SCN2A-RELATED DISORDERS
Leah F. Schust, Jennifer Burke, Christina SanInocencio, Brad A. Bryan, Karen S. Ho, Shawn M. Egan, 2024
Treatment Pipeline
FamilieSCN2A closely monitors the status of treatments for SCN2A-related disorders at all stages of the drug discovery pipeline, from discovery through approval. Some of these treatments are SCN2A-specific; others may help alleviate symptoms. The Foundation shares this pipeline for informational purposes only, without endorsement.
The graph below shows the status of known treatments in development as of December, 2025.
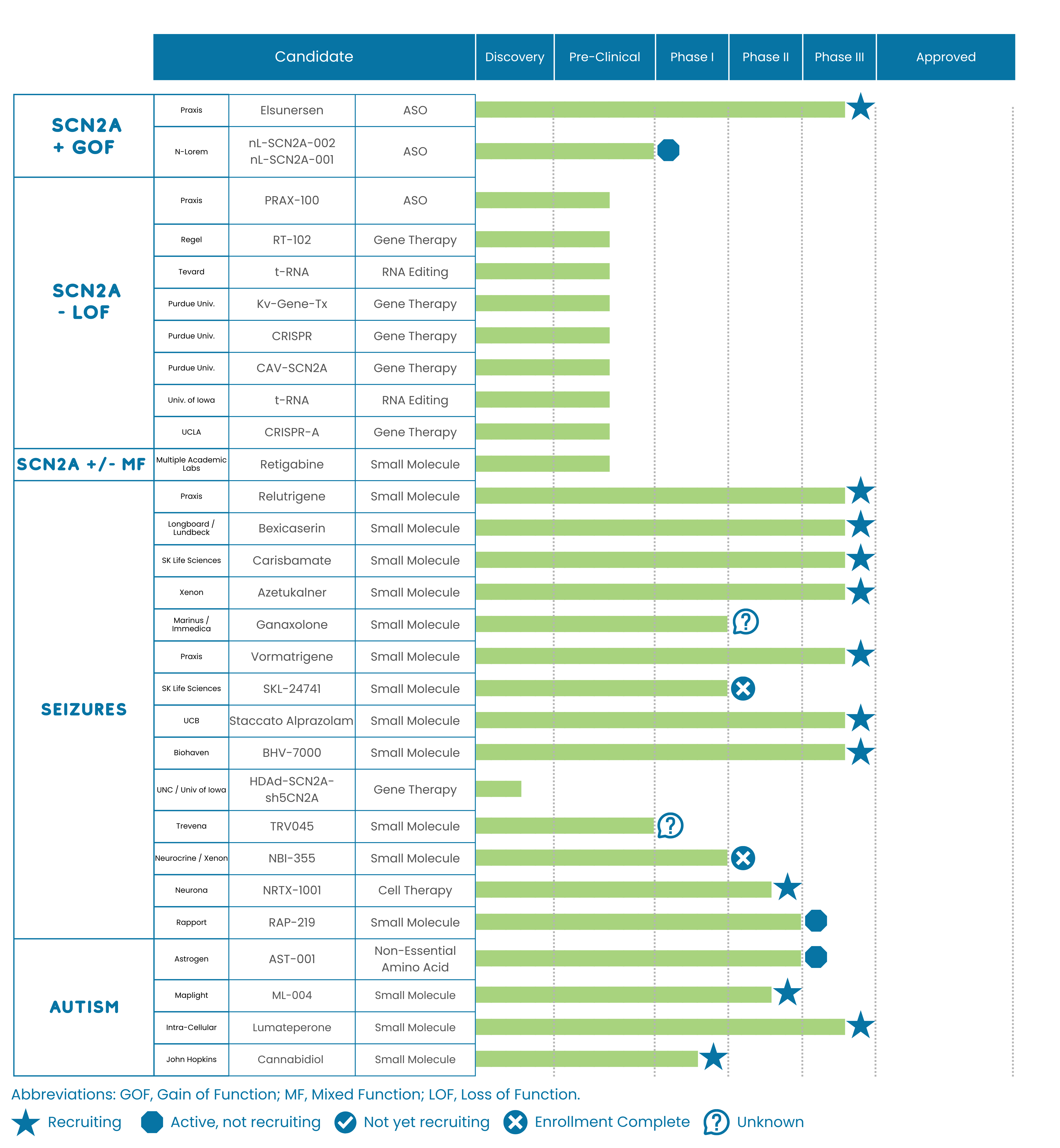
Studies Recruiting for Participants
To learn more about studies that are enrolling participants, please visit the Clinical Trials and Research Opportunities page of the FamilieSCN2A website.
The Drug Development Process
Drugs typically have to clear multiple clinical trial hurdles to support approval. The typical trial phases are 1, 2, 3, and 4 with lower number being earlier trials and larger numbers being more advanced trials. Phase 4 trials occur after the drug is approved by the FDA.
The Discovery stage includes conducting various tests to identify potential drug candidates for future therapy. It involves researching possible compounds before beginning tests in animals and cell cultures in the preclinical phase.
Before being tested on patients, a series of experiments is required to demonstrate that the prospective drug is both safe and effective. This stage of drug development is called preclinical (before the clinic/being used in patients). Preclinical experiments are often performed on cell lines in culture, small animal models, and with computer simulations.
Phase 1 trials are typically in healthy volunteers and are evaluating initial safety, and dosing in humans. These trials help ensure that the drug is safe enough to be evaluated in patients and also are used to identify an appropriate dosing range for subsequent trials.
Phase 2 trials are typically used to provide proof of concept (that the drug has an efficacy signal, is providing a benefit to patients) and also tests safety in the patient population. These trials often are used to help design and power registrational phase 3 trials.
Phase 3 trials are often the trials used to support the approval of the agent and may be the final clinical hurdle a drug needs to pass in order to be approved by the FDA.
Phase 4 trials take place after the FDA approves the drug for sale and marketing in the United States. They aim to further assess the drug's long-term effectiveness and safety. These trials are typically longer than previous trials and often evaluate additional indications.
To learn more about the drug development process, visit the How to Participate in Research section of the FamilieSCN2A website.
Types of Therapeutics in Development for SCN2A-Related Disorders

Small Molecule Therapy
Small molecule drugs make up most of the medications approved today. These are the types of medications you think of when you think of drugs (such as aspirin or ibuprofen). These medications target specific processes within the body and in the case of SCN2A, they are likely to target the sodium channel Nav1.2. Due to their small size, this class of medications can reach most areas of the body quickly and easily.
Genetic Medicines
1. Repeat-Dose Genetic Medicine: Antisense Oligonucleotide (ASO) Therapy
Antisense oligonucleotides (ASOs) are short DNA molecule drugs that interact with a patient’s RNA. ASOs are highly targeted and very specific drugs. Typically ASOs “silence” their target, meaning that they decrease expression of the gene (such as SCN2A) and thus decrease unwanted activity. Some ASOs have the potential to increase expression, or even correct the mutated gene. For neurological conditions, ASOs are typically administered intrathecally (via lumbar puncture) every few months. There are now multiple ASOs that have been approved by the FDA for various diseases.
2. Gene Therapy
Gene therapy refers to drugs that deliver genetic material to the patient. Typically these are one-time treatments, meaning patients are only treated once in their lifetime. This type of treatment can replace a dysfunctional gene that causes disease (such as SCN2A) with a healthy copy of the gene; inactivate or “knock out,” or “knock down” a mutated gene that is not working correctly; or, introduce a new gene into the body to help fight a disease.
3. Gene Editing
Gene editing is a form of therapy that often changes the patient’s genetic material (such as their DNA). This approach alters the genetic material of the patient by inserting, replacing, or deleting a DNA sequence. Some common types of gene editing that are being developed (not necessarily for SCN2A) include: Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR); Zinc Finger Nucleases (ZFNs); Transcription Activator-Like Effector Nucleases (TALEN); Base (DNA/RNA) Editing.
Emerging Therapies
To learn more about emerging therapies, please view the Cambridge Elements: SCN2A-Related Disorders publication or watch recordings on our YouTube channel.
