Participation in research is our greatest hope for better treatments and cures for all SCN2A-related disorders! To accelerate research, the best thing patients and their families can do is to learn about their SCN2A variant and available research projects. Here you will find educational resources on research and drug development processes, clinical trials, and SCN2A-specific projects. To stay up-to-date, please join our email list.
Therapeutic treatments & the drug development process
Small Molecule Therapy
Small molecule drugs make up most of the medications approved today. These are the types of medications you think of when you think of drugs (such as aspirin or ibuprofen). These medications target specific processes within the body and in the case of SCN2A, they are likely to target the sodium channel Nav1.2. Due to their small size, this class of medications can reach most areas of the body quickly and easily.
Genetic Medicines
1. Antisense Oligonucleotide (ASO) Therapy
Antisense oligonucleotides (ASOs) are short DNA molecule drugs that interact with a patient’s RNA. ASOs are highly targeted and very specific drugs. Typically ASOs “silence” their target, meaning that they decrease expression of the gene (such as SCN2A) and thus decrease unwanted activity. There are also some ASOs that have the potential to increase expression, or even correct the mutated gene. For neurological conditions, ASOs are typically administered intrathecally (via lumbar puncture) every few months. There are now multiple ASOs that have been approved by the FDA for various diseases.
2. Gene Therapy
Gene therapy refers to drugs that deliver genetic material to the patient. Typically these are one-time treatments, meaning patients are only treated once in their lifetime. This type of treatment can replace a dysfunctional gene that causes disease (such as SCN2A) with a healthy copy of the gene; inactivate or “knock out,” or “knock down” a mutated gene that is not working correctly; or, introduce a new gene into the body to help fight a disease.
3. Gene Editing
Gene editing is a form of therapy that often changes the patient’s genetic material (such as their DNA). This approach alters the genetic material of the patient by inserting, replacing, or deleting a DNA sequence. Some common types of gene editing that are being developed (not necessarily for SCN2A) include: CRISPR; Zinc Finger Nucleases (ZFNs); Transcription Activator-Like Effector Nucleases (TALEN); RNA editing.
There are currently no treatments approved specifically for SCN2A-related disorders. However, there are upcoming clinical trials and several treatments in preclinical stages. See SCN2A Research Projects & Treatment Pipeline.
Clinical Trial Updates
Patients are treated based on their symptom(s) and treatment is generally limited to medications approved by the FDA (in the United States). The effectiveness of treatments may vary depending on the type of SCN2A mutation present and how that mutation affects the SCN2A protein. How the SCN2A protein is changed is often classified into three categories and responsiveness to therapy may be predicted by these classifications:
- 1. A Gain-of-function (GoF) mutation leads to increased function of the Nav1.2 sodium channel protein. Conceptually, it would be as if the brakes were removed from a car, leading to the car going too fast.
- 2. A Loss-of-function (LoF) mutation leads to decreased function of the Nav1.2 sodium channel protein. Conceptually, it would be as if the engine was removed from a car, preventing it from moving.
- 3. A Mixed-function (MF) mutation leads to a change in function of the Nav1.2 sodium channel protein. Conceptually, it would be as if a car was only going 15 mph on a 65 mph speed limit highway and also didn’t stop at red lights.
For a more scientific detailed description see (M. Wolff et al 2017 and K. Bender et al 2018)
There are also drugs in clinical development (not approved for public use) that may be available to individuals enrolled in clinical trials. The benefits and risks of these drugs have not been fully characterized. These drugs have the potential for broader use if approved by regulators in your country.
Drugs in Preclinical Development
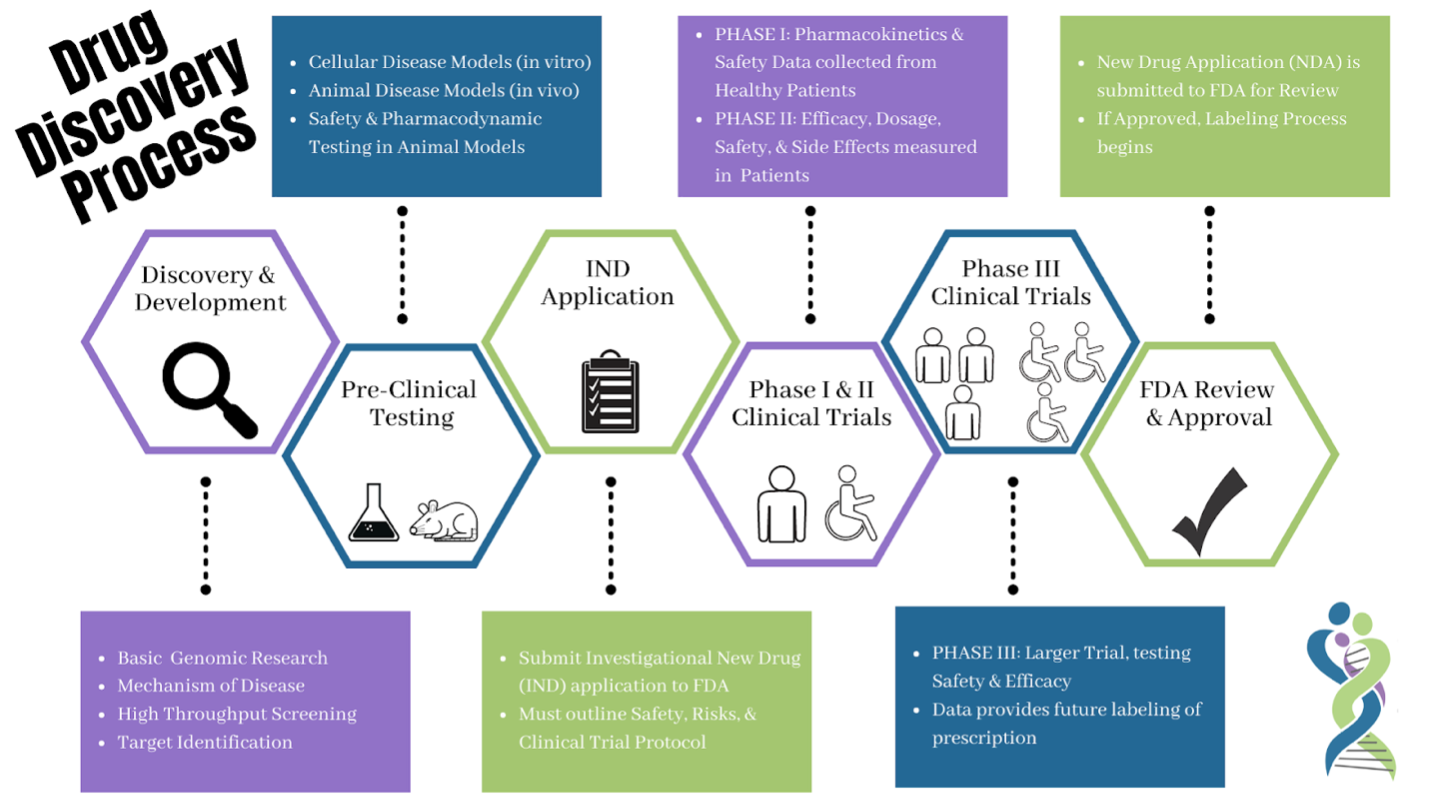
Before being tested on patients, a series of experiments are required to demonstrate that the prospective drug is both safe and effective. This stage of drug development is called preclinical (before the clinic/being used in patients). Preclinical experiments are often performed on cell lines in culture, small animal models, and also with computer simulations. Once a sufficient body of evidence is gathered (both efficacy and safety), the FDA may authorize an Investigational New Drug (IND) application which allows the medication to be used in clinical trials.
Drugs in Clinical Development
Clinical trials evaluate developmental medications and devices to determine whether they are both effective and safe. A series of trials are performed where patients are treated with the developmental drug or device and if these are successful the drug or device may become approved by regulators. Once approved, the drug or device becomes widely available for use by patients.
Learn more about the drug development process from the United States Food and Drug Administration.
Learn more about international rare disease drug development from the International Rare Diseases Research Consortium: IRDiRC REACT Orphan Drug Development Guidebook.