Positive Step Towards a Treatment for SCN2A Gain-of-Function Patients
Encouraging Initial Clinical Data for Praxis’ PRAX-222 in SCN2A Gain-of-Function DEE
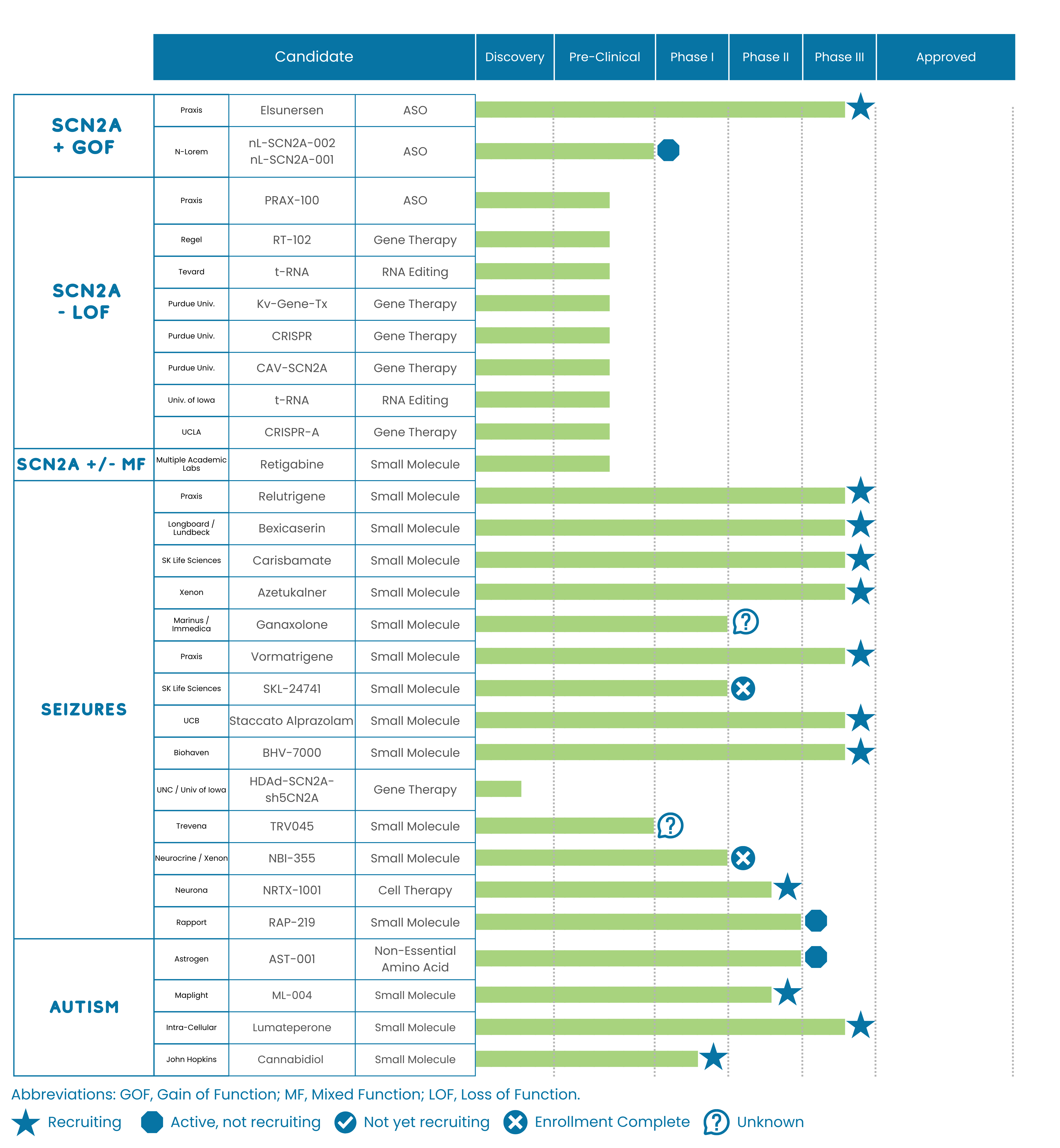
First in-human data for PRAX-222, an antisense oligonucleotide (ASO) in development for SCN2A gain-of-function was reported during a Praxis R&D day. This is the first clinical trial readout in SCN2A which represents an exciting milestone for our SCN2A community. To date, there have been 5 patients dosed with this therapy (4 patients in their clinical study EMBRAVE, and an additional newborn patient with a poor prognosis treated through expanded access). Praxis reported a seizure benefit in treated patients and didn’t disclose any treatment-emergent adverse events (TEAEs) or serious adverse events (SAEs) related to the study drug. Praxis plans to initiate a global pivotal study in 2024 after they compile and discuss their data with the FDA.
No Safety Signals is a Positive Signal
Initial clinical studies are designed to test and evaluate the safety and tolerability of the drug in development, and the initial safety profile of PRAX-222 was clean. PRAX-222 was well tolerated by patients, and there were no treatment-emergent adverse events or serious adverse events related to the study drug disclosed by Praxis. Furthermore, the patient treated under expanded access had the longest treatment exposure (7 doses, monthly treatment) and also experienced no serious adverse events.
Seizure Reductions Reported in Each Patient Treated
There was a 44% median seizure reduction following three doses of PRAX-222 (monthly intrathecal administration) in EMBRAVE with benefits also being observed in the percentage of seizure-free days experienced by the patients. Additionally, the patient treated under expanded access had their status epilepticus cease, experienced a reduction seizure frequency, and is now clinically stable. This data is still early (the number of treated patients is small and there is no control arm as a baseline-) but these are encouraging initial data for PRAX-222 and the SCN2A gain-of-function community.
Efficacy at 1 mg Dosing Would Be a Win
1 mg is a relatively low dose for an ASO (with many CNS programs dosing 10x to 100x more than this). Efficacy at a lower dose would be a positive because: 1. It would have a higher probability of a strong safety profile. There is a general belief that lower dosing means lower probability of safety issues with ASO and with the functional heterogeneity of SCN2A mutations it may also give more flexibility to safely select patients; 2. It means there is a considerable amount of room to increase the dose to optimize the efficacy profile in patients that may need that; and 3. Efficacy in early studies may speed up time to approval and distribution to our community (vs. spending meaningful time titrating dose to find an efficacy signal).
Next Steps for the Program
Recruiting is closed for this portion of the trial but Praxis expects to expand the study to a global pivotal trial in 2024 after a meeting with the FDA to discuss this data and next steps.
For more on these results see our interview with the Praxis management team here and for more information on eligibility for the study see study site EMBRAVE.