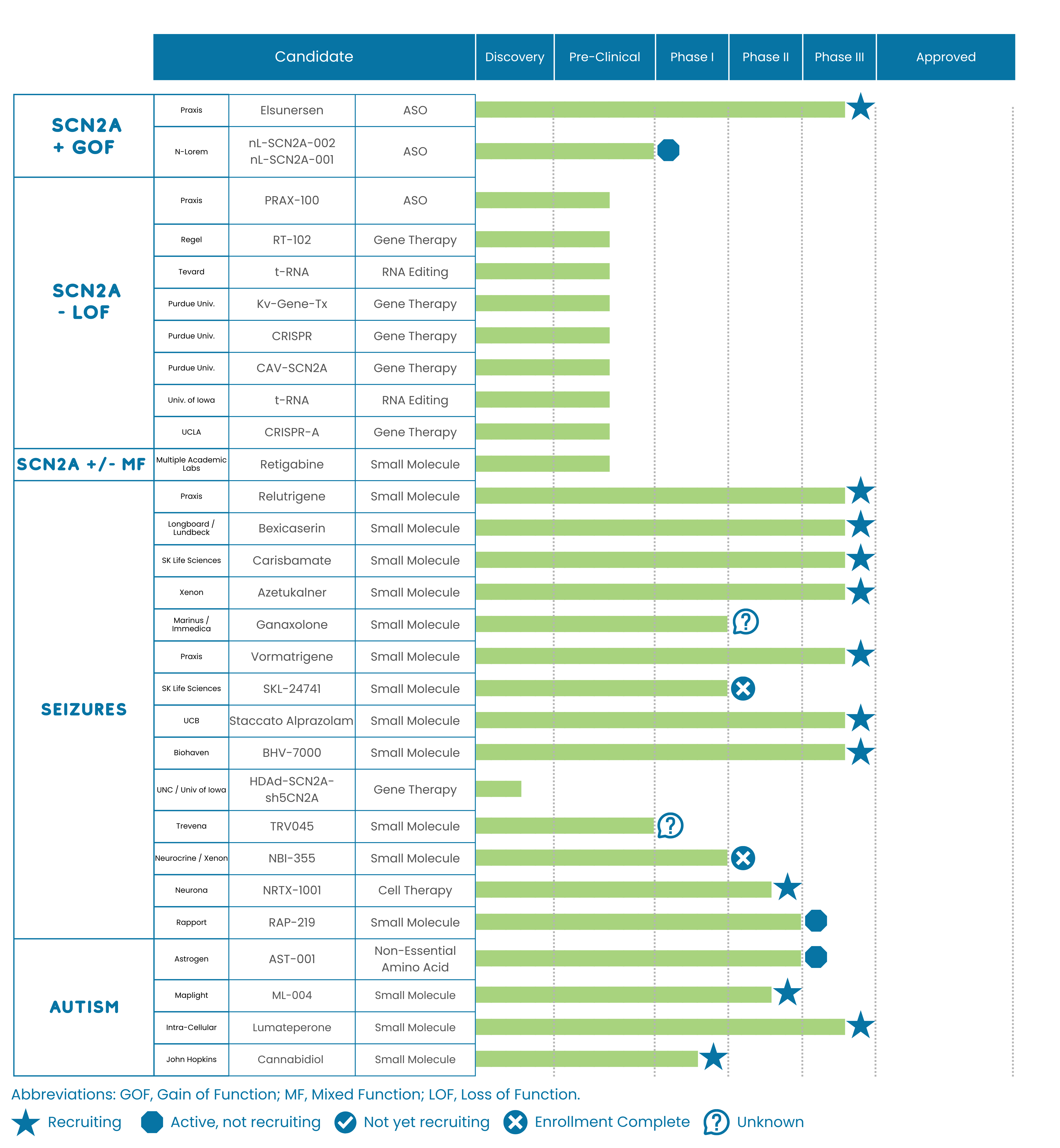
Praxis Precision Medicines, Inc updated our SCN2A community on the status of their three SCN2A programs in development and their CIITIZEN partnership.
PRAX-562
PRAX-562 is currently in phase 1 clinical trials (meaning it is being tested in humans). This agent has completed its single-ascending dose (SAD) portion of the phase 1 study and is now being evaluated in a multiple-ascending dose (MAD) portion of the trial. The SAD study was completed up to the maximum planned dose with no dose limiting toxicities (meaning the safety profile was good) and Praxis is currently at the highest preplanned dose in the MAD study with plans to dose escalate further if it continues to be well tolerated. Praxis plans to present initial PRAX-562 proof-of-concept data in mid-2021.
PRAX-562 is a small molecule inhibitor medicine that acts as a persistent sodium current blocker. Praxis is developing this agent in SCN2A and SCN8A and it has the potential to be therapeutic for individuals that have gain-of-function mutations. PRAX-562 has been granted the Rare Pediatric Disease Designation by the FDA.
PRAX-222
PRAX-222 is currently in IND-enabling, GLP (good laboratory practices) toxicology studies (these are pre-clinical/being tested in animals and not current being tested in humans). If successful, Praxis plans to file an IND (Investigational New Drug) application with the FDA in 2022 and move the drug into human clinical testing.
PRAX-222 is an antisense oligonucleotide medicine that acts to block the production of SCN2A protein. Praxis is developing this agent in SCN2A and it has the potential to be therapeutic for individuals that have gain-of function mutations. PRAX-222 has been granted both the Rare Pediatric Disease Designation and Orphan Drug Designation by the FDA
Loss-of-Function Program
This program is in collaboration with the Florey Institute, and is currently in discovery studies (meaning the drug is in early design and preclinical studies, and not currently being tested in humans. The Praxis team indicated that this program is still a few years away from clinical studies (being tested in patients).
This program is an antisense oligonucleotide medicine that is in development for loss-of-function SCN2a patients.
CIITIZEN
US SCN2A families have access to the CIITIZEN platform (see links below for easy access, either link will take you to the correct location). CIITIZEN is a healthcare technology company that gathers the complete medical records (labs, doctor notes, EEGs, imagining) of enrolled participants into a single portal. Information can be accessed by patients/caregivers/and providers that have been granted access by the caregiver.
Praxis has partnered with CIITIZEN to guide the development of biomarkers and to advance the understanding of SCN2A