The FamilieSCN2A Team: Amanda Gale – Program Manager, Angie Weaver – Director of Philanthropy & Development, Leah Myers – Executive Director, Jeff Cottrell – Interim Chief Scientific Officer, Melody Kisor – Director of Advocacy, and Morgan Weberg – Research Coordinator
Dear SCN2A Community,
Our team just returned from a wonderfully successful trip to the American Epilepsy Society (AES) conference in Atlanta. Our foundation had an educational booth where we connected with thousands of providers, advocacy groups, researchers, and drug developers. We forged new connections, strengthened existing collaborations, and learned of new developments and updates on treatments of interest to the SCN2A community. We are putting together a detailed recap of the meeting (expected at the end of the week), but wanted to first share some updates on clinical trials of interest.
Updates to the SCN2A Drug Development Pipeline
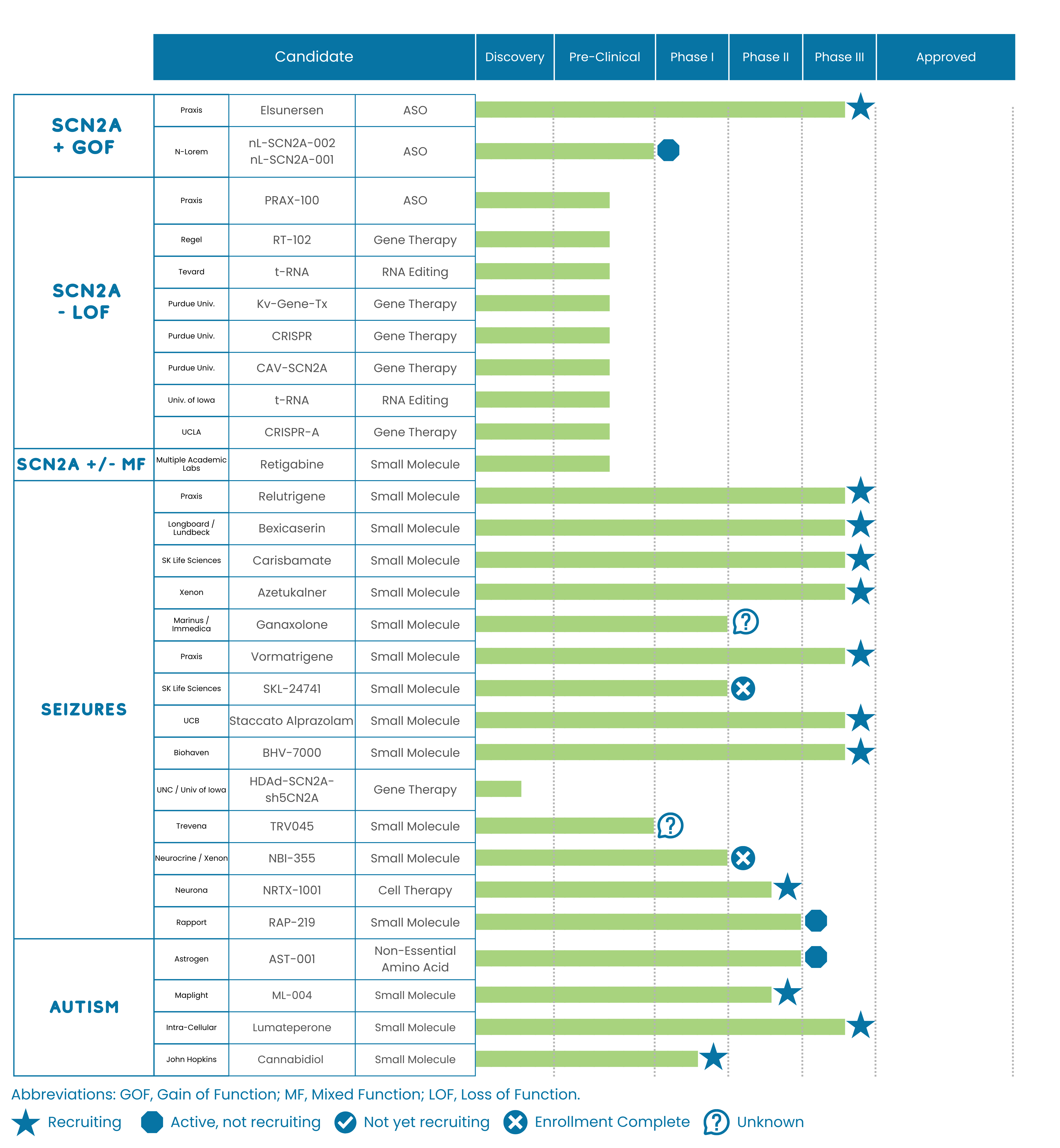
At the conference, drug developer Praxis Precision Medicines presented updates on several treatments in development, including:
- Relutrigine (PRAX-562), a small molecule drug in clinical trials for SCN2A-DEE and SCN8A-DEE.
- Elsunersen (PRAX-222), an Antisense Oligonucleotide (ASO) treatment in clinical trials for Gain of Function (GoF) variants of SCN2A.
In addition to presenting at the conference, Praxis also put out several press releases in the past few weeks, including updates on meetings with the Food and Drug Administration (FDA) for both treatments in development. Praxis presented results on the EMBOLD study (Phase 2/3) for Relutrigene (PRAX-562), demonstrating a 53% placebo-adjusted reduction in seizures (p<0.0002). This means that the study showed participants who received the treatment had approximately 50% fewer seizures compared to those who did not receive it. Additionally, the results showed a 66% increase in motor seizure-free days (p<0.0340) for participants who received the treatment. Finally, the results presented display a continued, well-tolerated safety profile with mild to moderate treatment-emergent adverse events. Side effects included pyrexia (fever), upper respiratory infection, somnolence (sleepiness), irritability, diarrhea, constipation, cough, and vomiting. In light of these results and their meetings with the FDA, Praxis ended the EMBOLD clinical trial earlier than anticipated, and updated the timeline to file a New Drug Application for Relutrigene (PRAX-562) in early 2026.
Additionally, Praxis presented updates to the clinical trial design for the EMBRAVE3 study (Phase 3) for Elsunersen (PRAX-222). The current study design has been converted to remove the placebo arm and will allow all study participants to receive the treatment in development, Elsunersen (PRAX-222). Additionally, Praxis presented an update on the EMBRAVE study (Phase 1/2) for Elsunersen (PRAX-222), announcing the release of topline results in the first half of 2026. Topline results are a big-picture view of whether or not a treatment in development was safe and worked (efficacy). Later on, drug developers release full results which include more details, deeper analysis, and longer-term data.
In addition to the updates presented by Praxis, a variety of other drug developers, researchers, and scientists presented updates on their ongoing SCN2A work.
- Parry Spratt, PhD, and his team at Regel Therapeutics presented data from their ongoing work on RT102, a targeted gene therapy aimed at restoring SCN2A haploinsufficiency (Loss of Function) in the preclinical stage.
- Olivia Kim-McManus, MD, from the University of California San Diego, presented updates on two participants with SCN2A-DEE treated with individualized antisense oligonucleotide (ASO) therapies created by the n-Lorem Foundation.
- A poster from Albert George, MD, a member of the FamilieSCN2A Foundation Medical and Scientific Advisor Board (MSAB), highlighted efforts to predict the effect of newly identified SCN2A variants.
- Morgan Robinson, PhD, member of the Yang Yang lab at Purdue University and a recent recipient of an Action Potential grant, presented results on using base editing on mini-brains made from human stem cells with specific SCN2A variants.
- Manoj Patel, PhD, and his team at the University of Virginia plan to initiate work to develop a base editor gene therapy treatment for SCN2A in the preclinical phase.
- We had multiple 1:1 meetings with other groups who are interested in accessing the Dragonfly registry database for the establishment of clinical trials for new treatment candidates in the future.
FamilieSCN2A Foundation is committed to Patient-Centered Drug Development
The FamilieSCN2A Foundation stands united with the global community in our shared desire for urgent, safe, and effective treatments for SCN2A-related disorders. While we await FDA review and assessment of the data collected in the clinical trials, the Foundation would like to take a moment to share how we can all pitch in to support Patient-Centered Drug Development efforts.
We are actively monitoring the status of all known SCN2A drug development efforts
The Foundation has robust scientific expertise in our TASCO and Medical & Scientific Advisory Board, and collaboration with a global research network. Our Chief Scientific Officer, Jeff Cottrell, and Research Coordinator, Morgan Weberg, closely monitor SCN2A research efforts across all stages of drug development. Please visit the Research Roadmap and Treatment Pipeline page of our website to see the most current status updates.

The most important thing families can do right now is to contribute to the Dragonfly Global SCN2A Registry and other Natural History Studies
When evaluating new treatments, the FDA relies heavily on natural history data to know how people with SCN2A-related disorders feel and function. The Dragonfly Study will provide critical information for regulators. Learn more by visiting the study website at https://scn2a.iamrare.org.
Please visit the Clinical Trials and Research Opportunities page to learn about other research that has or will contribute critical information for drug development, including Simons Searchlight natural history study, the Clinical Trial Readiness Study (CTRS), Citizen Health retrospective natural history study, and Clinical Picture Maker.
Please take 3 minutes to create a Clinical Research ID (CRID) to help link your participation in multiple studies. This will help the FDA get the complete picture for individuals who have generously contributed data from multiple sources. Learn more about the CRID on the How to Participate in Research page on our website, or go directly to https://thecrid.org/.
We have submitted a formal letter of intent to the FDA to host an Externally-led Patient-Focused Drug Development meeting
On August 28th, 2025, the FamilieSCN2A Foundation formally submitted a letter of intent requesting to host an Externally-led Patient-Focused Drug Development meeting with the US Food and Drug Administration (FDA). The goal of this one-day meeting is to help share critical information for regulators evaluating New Drug Applications and requests for clinical trials. Families from around the world will have the opportunity to participate in a variety of opportunities to share their stories and experiences before, during, and after the meeting. We are still waiting to hear back from the FDA, and will share details with our families and advocacy partners as soon as we have more information. To learn more, please visit the EL-PFDD page on our website or email Director of Advocacy, Melody Kisor.
Generous donations allow the FamilieSCN2A Foundation to support groundbreaking research efforts
The FamilieSCN2A Foundation supports drug development and discovery efforts through sustained, strategic research funding. With the generous support of our donors, the Foundation has invested more than $7 million in research to advance the understanding and treatment of SCN2A-related disorders. These investments have helped catalyze groundbreaking work across basic science, translational research, and patient-centered initiatives.
Funded studies include work from the Bender Lab at UCSF, focused on developing new biomarkers for SCN2A loss-of-function, and research from the Yang Lab at Purdue, which demonstrated that SCN2A variants impact the function of microglia—the immune cells of the brain. Publications from these and other Foundation-funded projects will help inform the FDA’s evaluation of future drug and product applications.
You can stay current on pivotal papers by visiting the Key Publications page on our website. We are most proud of the Patient-Centered Research section, which highlights the research that came directly from family participation in research. To learn more about how to contribute to the ongoing research funding efforts, visit our donation page or contact Director of Philanthropy and Development, Angie Weaver.
Closing sentiment
As a community, we know that progress in rare diseases does not happen overnight. It is built carefully — through rigorous science, meaningful partnership with regulators, and the steady participation of families who share their experiences to move the field forward. Every data point, every story, and every act of engagement helps shape the path toward safe and effective treatments.
The FamilieSCN2A Foundation remains deeply committed to patient-centered drug development and to ensuring that the voices of individuals and families affected by SCN2A-related disorders are reflected throughout the process. While there is still important work ahead, the momentum we are seeing is real — and it exists because of this community’s dedication, generosity, and hope.
Thank you for continuing to show up for one another, to support research, and to believe in a better, brighter future for all SCN2A families.
With gratitude,
FamilieSCN2A Foundation Team
Amanda Gale – Program Manager
Angie Weaver – Director of Philanthropy & Development
Leah Myers – Executive Director
Jeff Cottrell – Chief Scientific Officer
Melody Kisor – Director of Advocacy
Morgan Weberg – Research Coordinator


