What to Expect When Considering a Clinical Trial: Clinical Research 101
Thinking about participating in a clinical trial, but not sure what it really means for your child and your family?
Learn What to Expect When Considering Participating in a Clinical Trial
On July 20th, 2025, the FamilieSCN2A TASCO (Team for Accelerating Science and Clinical Outcomes) hosted a special fireside chat to walk through the basics of clinical research. Using a unique role-play format, the panel featured:
- Karen Ho, as the Drug Developer
- Shawn Egan, as the Clinician
- Morgan Weberg, as the Caregiver/Patient Perspective
The team discussed what clinical trials are, how they work, what families can expect during participation, and how research integrates into the overall care process. You can view a recording of this Town Hall here: What to Expect When Considering a Clinical Trial.
What is Clinical Research?
Clinical research is the scientific process of studying health and disease in people. It’s how we discover:
- Which treatments are safe
- Which treatments are effective
- How diseases progress over time
Clinical research is crucial for advancing medical knowledge, creating new treatments, and improving patient care. Unlike routine clinical care, it primarily involves collecting data to guide treatment decisions for a specific diagnosis or symptoms rather than managing a patient’s overall care. It is vital to determine your personal goals in clinical trial participation and to talk about clinical research options with your child’s healthcare provider.
Two Main Types of Clinical Research
Observational (Non-Interventional) Studies:
These studies track symptoms and outcomes without any experimental treatment. They include:
- Patient registries
- Natural history studies
- Trial readiness research
Families may fill out surveys, questionnaires, record videos, or complete assessments that help researchers better understand SCN2A-related disorders over time.
Interventional Clinical Research Trials:
These studies test a treatment, like an experimental (also called “investigational”) new drug, gene therapy, or device. They help answer:
- Is it safe?
- Is it effective and helpful (efficacy)?
- What side effects might occur?
A more effective drug has a higher likelihood of producing the intended effect. Various tests, such as MRI scans, blood tests, or seizure diaries, help assess the drug’s impact and safety profile. Clinical trials are FDA-regulated, and interventional trials require more intensive medical monitoring. Ultimately, this data supports the FDA approval process for a new treatment.
How Do Clinical Trials Work?
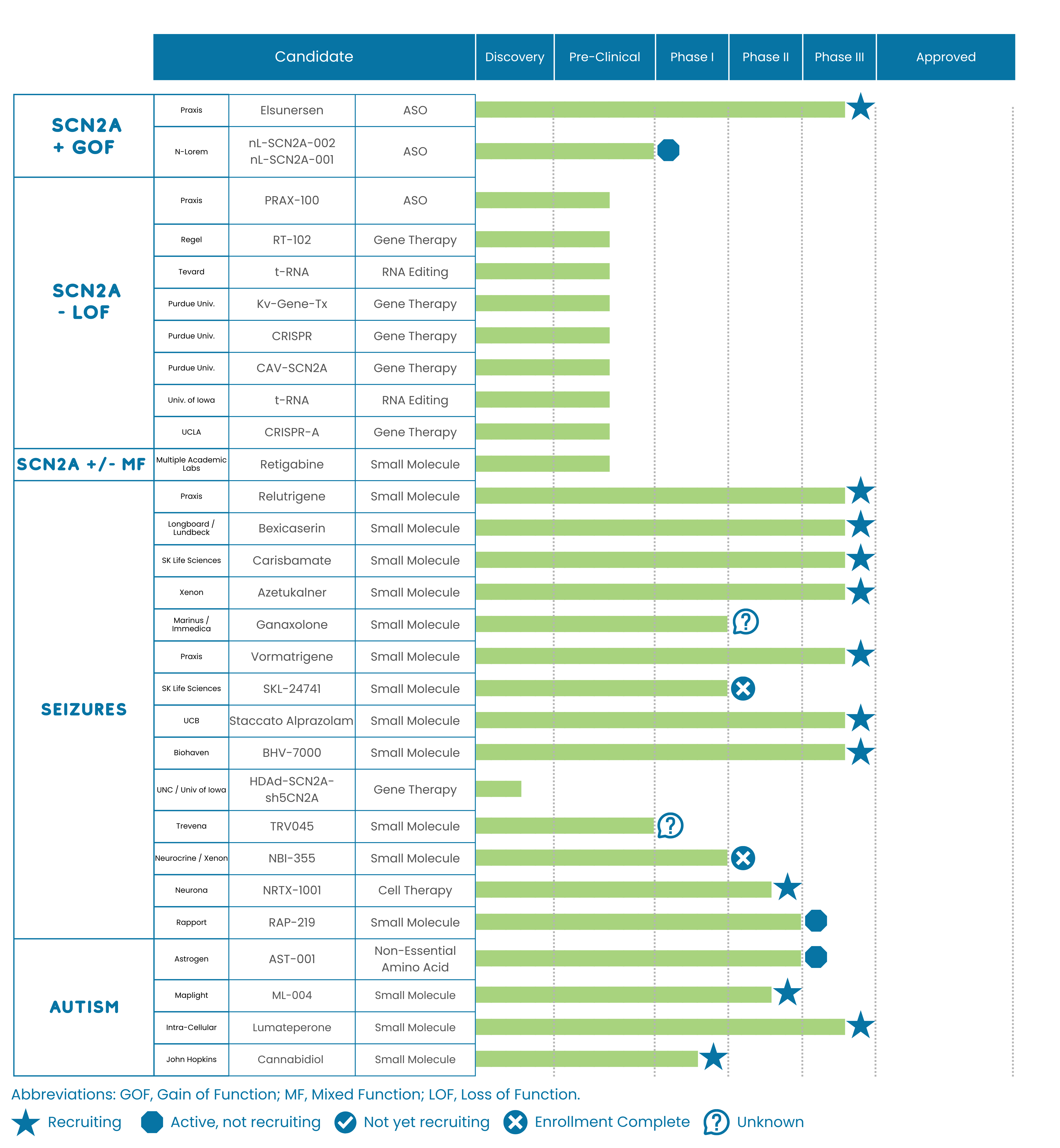
Drugs typically have to clear multiple clinical trial hurdles to support approval. The typical trial progresses through phases 1, 2, 3, and 4. In rare diseases, some trials may combine phases. Details about each stage of drug development are given below.
- Preclinical – Before being tested on patients, a series of experiments is required to demonstrate that the investigational drug is both safe and effective. Preclinical experiments are often performed on cell lines in culture and in animal models.
- Phase 1 – These trials are either conducted in healthy volunteers or in patients, and are used to evaluate initial safety and dosing levels in humans.
- Phase 2 – These trials are typically used to provide proof of concept that the drug has an efficacy signal that provides benefit to the patient population, while continuing to test safety.
- Phase 3 – These trials are often larger and are used to support the approval of the investigational drug by the FDA.
- Phase 4 – These trials take place after FDA approval has been granted to a drug. While they are not always required by the FDA, if they are conducted they aim to further assess the drug’s long-term effectiveness and safety by collecting “real world evidence.” These trials are typically longer than previous trials and may evaluate additional indications or additional safety signals.
Each step helps ensure a treatment is safe and effective before it reaches patients more broadly.
What Are “Drug Modalities”?
Different kinds of ‘drug modalities’ exist, referring to the chemical nature of the drug and how it is delivered. Some examples being studied or developed for SCN2A-related disorders include:
Neuromodulation Devices – Implants that adjust brain activity (e.g., Vagus Nerve Stimulation (VNS),Responsive Neurostimulation (RNS) Or Deep Brain Stimulation (DBS)).
Small Molecules – Traditional drugs that target the sodium channel (Nav1.2). They can be delivered in a number of ways, including orally, via g-tube, or by injection.
Repeat-Dose Genetic Medicine – Antisense Oligonucleotides (ASOs) are short DNA/RNA drugs that silence or boost gene activity. (For neurological disorders, they are usually delivered via injection to the brain or spinal cord.)
Gene Replacement Therapy – Delivers a healthy copy of a gene (These are typically delivered as a one-time injection to the brain or spinal cord.)
Gene Editing (CRISPR, TALEN, ZFN, Base Editing) – Fixes or alters DNA inside the body. (These are also typically delivered as an injection to the brain or spinal cord.)
Cell Therapy – Administration of modified or donor cells to induce benefit by delivering therapeutic cells directly into the body
What Trial Participation Might Look Like
Joining a clinical trial is a commitment—but understanding the process helps. It is important to know that you can choose to exit a study at any time. Clinical trial participation is voluntary. Here’s what most families can expect:
- Screening & Eligibility: Your child must meet specific criteria to be eligible to participate in a clinical trial. There are eligibility requirements that a participating clinician at a clinical trial site will screen for to determine eligibility. Examples include the presence of the disorder, disease, or symptom being treated, age, extent of developmental delay, clinical seizure phenotype, frequency, presentation, and medical history. These requirements are typically labeled as inclusion or exclusion criteria and are generated by the drug developer. A participant must meet all of the inclusion criteria and have none of the exclusion criteria in order to be enrolled in a trial.
- Consent to participate: Once your child is determined to be eligible to participate in a trial, an informed consent or assent form must be signed by the participant or designated parent/guardian. It is important to understand any document being signed.
- Randomization: The participant will be randomly placed into a group based on the study design. For example, a patient could have an equal chance of being randomized into the active drug group or placebo group, referred to as a 1:1 ratio. Different trials can have different randomization ratios depending on the trial’s design. When your child is randomized into a group, you and the clinician at the trial site will typically not know which group they are randomized into. Typical trials are labeled as Double-Blind, Placebo-Controlled, Randomized Clinical Trials, meaning that neither the patient, their family, nor the clinical trialist knows if an active drug or a placebo was received.
- Treatment Period: Once randomization is complete, the trial begins, and the treatment period continues. Your child receives the active drug (or placebo) for a predetermined period of time, depending on the trial design. Throughout the treatment period, there will be patient visits to the clinical trial site. During these visits, your child will undergo a physical exam, vital sign assessment, and might receive blood draws and other tests to record data that will be used to evaluate how effective and safe the drug is.
- Follow-Up: At the end of the treatment period, a follow-up patient visit occurs for a final safety assessment and either to complete participation in the trial or provide the option to enroll in an open-label extension or expanded access program (if available). If you decide to have your child complete and exit the trial, a final physical exam, vital sign assessment, and other tests will be conducted at the last patient visit. If you decide to have your child continue via Open-Label Extension (OLE) or join an Expanded Access Program (EAP), you will know that your child is receiving an active drug. Additional clinical visits and safety assessments will then be ongoing until the OLE or EAP ends.
Watch the Full Town Hall
Recorded July 20, 2025, Click Here: What to Expect When Considering a Clinical Trial.
If you’ve ever felt confused or curious about clinical trials, this session is a great place to start.
You’re Not Alone
Navigating an SCN2A-related disorder is hard enough — navigating research shouldn’t be. FamilieSCN2A and the TASCO team are here to empower and equip families as true partners in research.
Whether you’re ready to participate or just gathering information, your journey matters — and your voice matters.
Need Help Understanding a Trial?
The FamilieSCN2A team is here to answer questions and point you toward trusted resources. Reach out anytime — we’re in this together.
For More Information:
Visit: https://www.scn2a.org/research/
Contact research@scn2a.org for more information.
Abbreviations: CRISPR, Clustered Regularly Interspaced Short Palindromic Repeats; FDA, Food and Drug Administration; MRI, Magnetic Resonance Imaging; TALEN, Transcription Activator-Like Effector Nucleases; TASCO, Team for Accelerating Science and Clinical Outcomes; and ZNF, Zinc Finger Nucleases.