Patient-Centered Drug Development
Learn how FamilieSCN2A Foundation engages in patient-centered drug development by amplifying the patient voice with regulatory agencies and industry partners.
Patient-Centered Drug Development is an intentional effort to ensure that patient needs and priorities are meaningfully included at all stages of clinical research and development.
Regulatory and industry partners play critical roles in the treatment development process. Learn how FamilieSCN2A engages in patient-centered drug development by amplifying the patient voice with regulatory agencies and collaborating with industry partners.
FDA Engagement
The U.S. Food & Drug Administration (FDA) actively engages with patient advocacy groups like FamilieSCN2A to ensure that the patient and caregiver voice is considered in their work. The FDA relies on the information shared by patients and caregivers to help them at all stages of the drug development process, including clinical trial design, outcomes assessments, and product approvals.
Here are some ways that FamilieSCN2A has engaged with FDA:
2025
FamilieSCN2A Foundation submitted a letter of intent to host an Externally-led Patient Focused Drug Development (EL-PFDD) meeting with the FDA. The goal of this public meeting is to share the critical patient voice for future drug development efforts. Stay tuned for updates.
2022
Chief Scientific Officer Shawn Egan, PhD and his daughter Harper share A Rare Disease Story for FDA Rare Disease Day awareness:
“Harper's life has really been a roller coaster that's really driven by seizures. (...) But we're hopeful and optimistic and so proud of Harper because she continues to surprise us and the doctors on her strength and fortitude. And we're encouraged about the progress that industry and science is making to try to bring something transformative to Harper.”
2021
FamilieSCN2A Foundation hosted a Patient Listening Session with FDA to share critical information for future drug development efforts.
Industry Partners
The FamilieSCN2A Foundation works closely with biotech and pharmaceutical partners to help guide and support drug development. The foundation also invests considerable resources to de-risk working on SCN2A, making it more likely that industry partners will start and complete drug development programs.
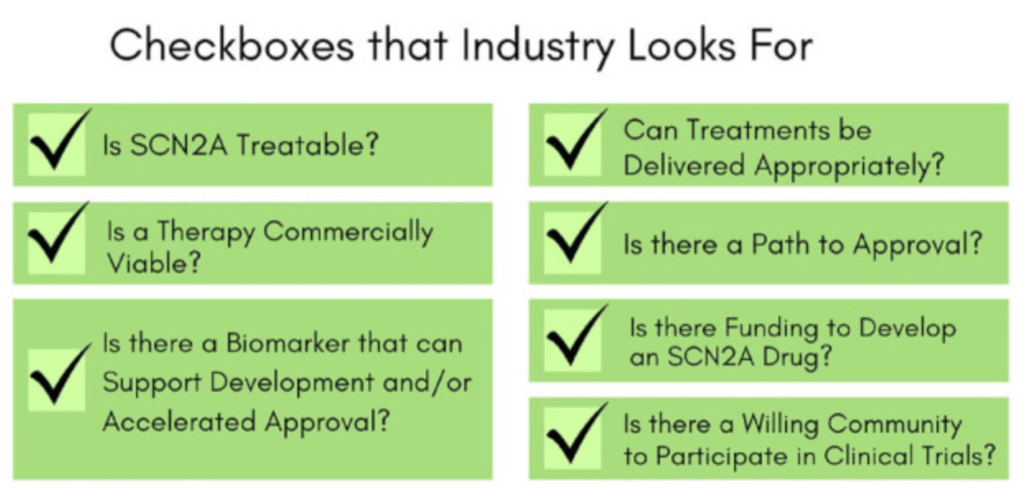
Advocacy efforts include:
Robust community engagement and education
Sponsoring a patient registry, natural history studies, and a clinical trial readiness study
Recruiting for research and clinical trial opportunities
Sharing data, including with the C-Path RDCA-DAP
Funding and supporting research at all stages of development, including biomarkers and clinical trial endpoint development
Uniting a strong international research network of advisors
Hosting conferences and scientific convenings
Engaging with the FDA – early and often!
Collaborating with advocacy partners
Nurturing industry relationships
Learn more by watching videos on The FamilieSCN2A Foundation industry video playlist
FamilieSCN2A Foundation
Policies on Engaging with Industry and Regulatory Partners
Corporate Relations
Participation in Food and Drug Administration Hearings and Meetings
Timely Requests
