How to Participate in Research
Everything you need to know
about participating in research
Everything You Need to Know About Participating in Research
Participation in research is our greatest hope for better treatments and cures for all SCN2A-related disorders! Get ready to participate in research by learning more about clinical trial basics, types of research, and participant rights, responsibilities, & protections. Decide if and when to participate, how to give informed consent, what your rights & responsibilities are as a research participant, and how to protect your identity and privacy.
Community Education Topics
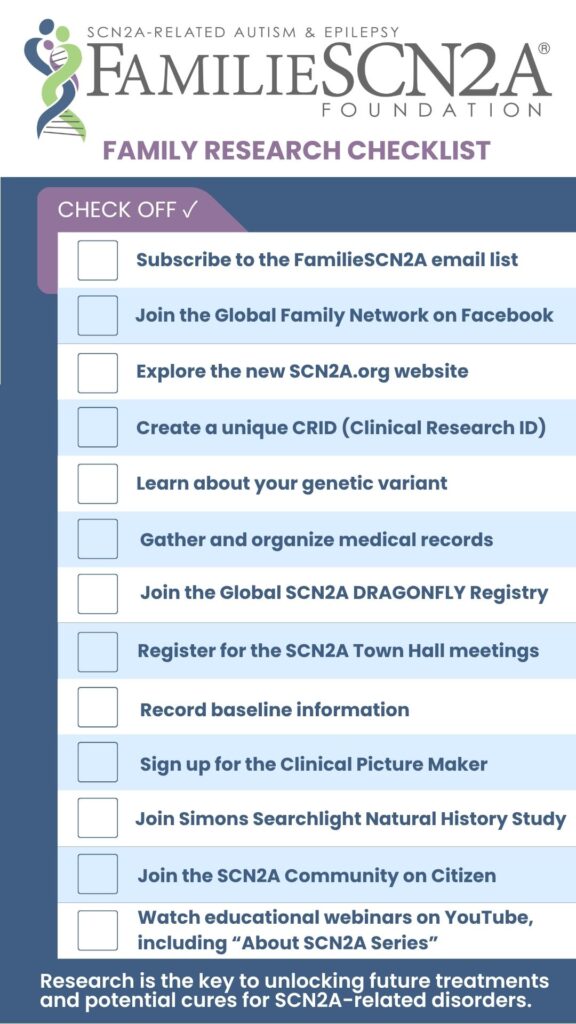
Even before you participate in research, there are some things you can do to make sure that you are ready when an opportunity comes up.
Stay informed
Subscribe to the FamilieSCN2A Foundation email list to receive up-to-date information about research opportunities.
Become familiar with your SCN2A genetic change or variants
Speak with your doctor and genetic counselor about your SCN2A genetic changes (also called “variants” or “mutations”). Ask lots of questions! Learn about genetic terminology, such as “gain of function” or “loss of function.” Use the tools and links provided on the FamilieSCN2A website, including educational videos and the glossary found in Family Education and Support.
Record baseline information and take videos
In order to determine if an intervention or treatment is effective, it can be really helpful to know how a person feels and functions before they try something new. You can record baseline information in lots of different ways, including using a symptom diary or journal, wellness apps, or wearable trackers. Taking photos and videos on your phone can be a wonderful way to document challenges and show changes over time. You may choose to store and share these videos with your medical team and researchers using Clinical Picture Maker.
Collect and organize medical records
Having copies of medical records can be extremely helpful for participating in research. You can request copies of your medical records directly from your provider, or if your medical provider has an electronic health record system (such as ‘MyChart’) you can download the information from there.
Once you have your records, you can choose to share them for research. If you have participated in Simons VIP/Searchlight, they can assist you with this process. If you have a Citizen account you can share information electronically with anyone you choose. For privacy, you may choose to redact or remove personal information from records before sharing with research.
Learn how to find research opportunities
There are several ways to learn about research opportunities of possible interest.
Your doctor may share information at a medical appointment, or reach out to potential participants when new opportunities come up. Sometimes your own doctor is also the researcher, or they may be helping to recruit for studies for another researcher.
There may be opportunities to participate in research at a different place or with a different doctor than the one who you usually work with. You may have to travel quite a distance for some research opportunities, whereas others may be local or remote.
You may learn about research opportunities from social media, internet searches, or other families. In that case, you may want to reach out to your doctor to discuss the research. They can help you understand if you meet the eligibility requirements, and can share their opinion about whether this is an opportunity that might be a good match for you. They may also be able to connect you with the trial sponsor or principal investigator of the research.
The National Library of Medicine maintains an international database of research opportunities called ClinicalTrials.gov. You can search for research studies related to “SCN2A” or specific related conditions (such as epilepsy or autism). The general information about each study, including locations and the primary contact person, is listed for each study.
If you learn of opportunities or are contacted to participate in research
FamilieSCN2A keeps a comprehensive list of clinical trial opportunities, but you may learn of others.
Please reach out if you learn of any opportunities available to people with SCN2A so that we can review and possibly share them with others. If you are unsure about the ethics involved or have not heard about the study through the FamilieSCN2A Foundation, please email our Research Coordinator. We can help you determine the legitimacy of the research, as well as ensure that all avenues to collaboration are open for the best interest of the community.
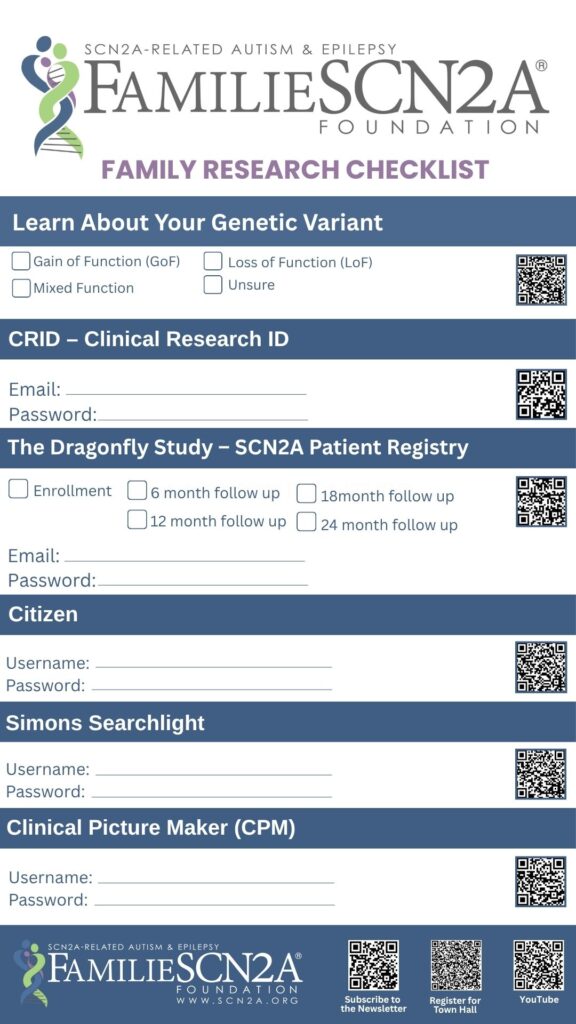
To protect your identity and allow researchers to securely connect data from different studies, FamilieSCN2A encourages all patients enrolled in research to create a Clinical Research ID (CRID). The CRID is a unique ID, assigned only to you, which you can choose to share with researchers. By sharing your CRID, researchers can then reuse, merge, and/or share your research data without needing your PII (Personal Identifiable Information) or PHI (Personal Health Information).
What is CRID?
CRID, or Clinical Research Identifier, is a unique identifier for patients participating in clinical research studies. This identifier is generated by the patient or their parent and serves as a secure and consistent way to identify a patient's information across multiple research studies.
Is CRID Secure?
Yes, CRID takes security seriously. All CRID data is stored on secure Amazon AWS servers and is encrypted both in the database and while in transit. Additionally, we do not use Google Analytics.
CRID Principles
- CRID is always free for the parent/patient
- CRID is always optional for research studies
- CRID data is private and will never be sold or shared with any third parties
What does a CRID look like?
A CRID identifier is an 8-character random string of letters and numbers that is unique to each patient.
How to use the CRID?
Patients can share their CRID identifier with investigators on research studies they are or have been enrolled in.
Why use CRID?
Many patients are unaware that their data is not shared with other researchers working on the same or similar diseases. They may also experience "survey fatigue" from being asked to complete similar forms for multiple studies. CRID allows for the sharing, reuse, and merging of data across studies by connecting deidentified patient information.
How do I create my CRID identifier?
To create a CRID identifier, visit the website https://TheCRID.org.
If you have any questions or concerns about CRID, or if you need assistance, you can send an email to info@thecrid.org.
There are many types of research studies, from basic science experiments in the lab, to patient registries and natural history studies, all the way to complex gene therapy clinical trials. This section will help you understand the most common types of research studies, with examples of actual studies of possible interest to people living with SRDs.
Before researchers are ready to do clinical trials, they have to spend many years working on preclinical research to understand basic biology, create animal and cell models, and test hypotheses. Preclinical research is an important part of the drug development process. People may be asked to help during this part of the process by donating blood or other tissue, or by sharing medical records or other information to help researchers understand more about SCN2A-related disorders.
The two major categories of clinical research that people may be asked to participate in are observational studies and clinical trials. Within each category, there are different types of studies that each contribute to the ultimate goal of finding safe and effective treatments for SCN2A-related disorders. Observational studies include patient registries, natural history studies, and clinical trial readiness studies. Clinical trials include testing interventions, such as an experimental new drug, gene therapy, device, or surgical procedure.

Source: National Library of Medicine https://clinicaltrials.gov/study-basics/learn-about-studies
Observational Studies
One of the most important ways to contribute to future drug development is by enrolling in a patient registry and/or natural study. These studies help researchers understand what is most important to patients and families, so that the treatments they design will be targeted to address those needs.
A patient registry is a collection of information about individuals, usually focused around a specific diagnosis or condition. Individuals provide information about themselves to a registry on a voluntary basis.
From NIH:
“In the context of therapy development, a patient registry (also called a disease registry) is a database that collects and stores information about patients diagnosed with a specific disease, genetic disorder, or medical condition.”
Examples of SCN2A patient registries:
- SCN2A Dragonfly Study (NORD IAMRARE platform)
- Simons Searchlight
Learn more about patient registries from the NORD video series:
Longitudinal Studies / Natural History Studies
In natural history studies, the goal is to see how a disorder progresses over time. They can follow people forward in time (prospective), or look backwards in their medical records to collect information recorded before the study (retrospective). Some patient registries also collect information that can be used for natural history studies. Insights from all of these types of observational studies can help drug developers and regulators design ways to measure successful interventions.
Examples of SCN2A natural history studies:
- Simon’s Searchlight (mixed patient registry / natural history study)
- Citizen Health Natural History Study (retrospective / chart review)
Learn more about natural history studies from the NORD video series.
Clinical Trial Readiness Studies
Some studies are designed to help researchers know what to measure to test if a treatment is working, such as clinical outcome assessments or endpoints. These studies could include identifying or developing biomarkers (like blood tests), or creating assessments that can show meaningful change in areas that are important to patients and families (such as improved communication or fine motor control).
Examples of clinical trial readiness studies:
- SCN2A Clinical Trial Readiness Study
- Inchstone Project
Supplemental research tools and studies
Some research studies and tools help to supplement patient registries and natural history studies. These projects include tools such as symptom diaries or wellness apps, wearable devices, and platforms to help you store medical records and videos. These tools can help you collect and contribute data to help researchers better understand what it feels like to live with SCN2A-related disorders. They may also help you collect and organize information that can be helpful to share with your medical team or teachers.
Examples of supplemental research tools:
- Clinical Picture Maker (to store and share videos)
- Citizen Health (to store and share medical records)
- Epsy (seizure tracking app)
- MyDataHelps (connects wearable data with symptom trackers; users may be eligible to enroll in research studies)
Drug Repurposing Studies
In this type of study, researchers are trying to find existing drugs or chemical compounds that may help a new condition. In some cases, these drugs are already FDA-approved for another condition, and could potentially be used for a new disorder. In other cases, the drug repurposing studies identify a chemical compound that could be further developed into an experimental drug. In either case, drug repurposing studies may identify potential new drugs faster than the traditional methods in the drug development process.
Examples of drug repurposing studies and projects:
- High-throughput screening of existing compounds as part of the partnership with Cellectricon
- EveryCure
Biobank / Biorepository and Human Tissue Studies
Some research studies involve the collection and storage of donated human samples, including blood, cerebrospinal fluid, skin cells or brain tissue. Sometimes the participant has the sample removed specifically to participate in research (such as with blood samples), and other times it is leftover from a surgical procedure and would otherwise be discarded (such as CSF or brain tissue from planned epilepsy surgeries).
Some people generously choose to donate the brains of someone who has died, with the hopes that researchers can learn more about SCN2A-related disorders. This heroic act allows the loved one to continue to contribute, even after they have passed away.
Examples of biorepositories or human tissue studies:
- Simons Searchlight - collects blood
- Rare Epilepsies & Brain Disease tissue bank - collects brain tissue and CSF samples during planned epilepsy surgeries
- Brain tissue collection after a person has died (postmortem)
- NIH NeuroBank
- Autism BrainNet - collects brain tissue from people with ASD
- List of other brain tissue biobanks from DEE-P Connections
Interventional Studies
When many people think of research studies, they are often thinking of interventional or experimental studies. In these types of studies, researchers are testing an intervention to see if they can change how a person feels, functions, or survives.
Examples of interventions:
- Drug or medication
- Exercise or physical therapy
- Surgical procedure or device
- Behavioral change
Clinical Trials
Clinical trials focus on testing an experimental drug or device with the goal of getting it approved by FDA or other regulatory agencies. Depending on how the clinical trial is designed, the participant may receive the experimental treatment, a placebo (inactive substance), or both. To learn more about clinical trials, including current opportunities to participate, visit the research pages of the FamilieSCN2A website.
Examples of clinical trials include:
- The EMBOLD Study
- The EMBRAVE Study
- LGS Discover Study
- X-TOLE2/X-TOLE3/X-ACKT studies
- Cannabidiol study for Adults with ASD
Personalized Medicine / Precision Medicine Trials (N=1)
These highly personalized trials are sometimes called “N of 1” or “N=1” because the number of participants in the study is just one. (Sometimes a few additional people will be included in the trial and it is called “N of few”). Success is measured by changes in a person’s baseline and/or predicted course of the disorder, rather than against other participants.
Examples of personalized medicine trials include:
- Individualized ASO (Antisense Oligonucleotide) therapy from n-Lorem for targeted SCN2A mutations.
What to Expect When Considering a Clinical Trial
Learn all about the clinical trial process in this recording of the July 20, 2025 FamilieSCN2A Town Hall meeting.

From NIH:
“Clinical trials are part of clinical research and at the heart of all medical advances. Clinical trials look at new ways to prevent, detect, or treat disease. Clinical trials can study:
- New drugs or new combinations of drugs
- New ways of doing surgery
- New medical devices
- New ways to use existing treatments
- New ways to change behaviors to improve health
- New ways to improve the quality of life for people with acute or chronic illnesses.
The goal of clinical trials is to determine if these treatment, prevention, and behavior approaches are safe and effective. People take part in clinical trials for many reasons. Healthy volunteers say they take part to help others and to contribute to moving science forward. People with an illness or disease also take part to help others, but also to possibly receive the newest treatment and to have added (or extra) care and attention from the clinical trial staff. Clinical trials offer hope for many people and a chance to help researchers find better treatments for others in the future.”
Source: https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics
Additional Resources
Videos from US Department of Health and Human Services
Part 1: What is medical research?
Part 2: Deciding to participate in clinical trials
Part 3: Questions to ask before volunteering in clinical trials
Part 4: Explaining randomization in clinical trials
Part 5: How is medical research different from medical care?
NIH Clinical Trial Basics
https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics
- What are clinical trials and why do people participate?
- What are clinical trials and why would I want to take part?
- Why is diversity and inclusion important in clinical trials?
- How does the research process work?
- What are clinical trial protocols?
- What is an Institutional Review Board?
- What is a clinical trial sponsor?
- What is informed consent?
- What are the types of clinical trials?
- What are the phases of clinical trials?
- What do the terms placebo, randomization, and blinded mean in clinical trials?
- Who takes part in clinical trials?
- What do I need to know if I am thinking about taking part in a clinical trial?
- What questions should I ask if offered a clinical trial?
- How is my safety protected?
- What happens after a clinical trial is completed?
- How does clinical research make a difference to me and my family?
DEE-p Connections ABC's of Clinical Trials
Global Genes has created a helpful toolkit for families considering participation in a clinical trial: click here.

There are currently no treatments approved specifically for SCN2A-related disorders. However, there are upcoming clinical trials and several treatments in preclinical stages. Learn more on the SCN2A Research Roadmap & Treatment Pipeline page.

Types of Therapeutics in Development for SCN2A-related disorders
Small Molecule Therapy
Small molecule drugs make up most of the medications approved today. These are the types of medications you think of when you think of drugs (such as aspirin or ibuprofen). These medications target specific processes within the body and in the case of SCN2A, they are likely to target the sodium channel Nav1.2. Due to their small size, this class of medications can reach most areas of the body quickly and easily.
Genetic Medicines
1. Repeat-Dose Genetic Medicine: Antisense Oligonucleotide (ASO) Therapy
Antisense oligonucleotides (ASOs) are short DNA molecule drugs that interact with a patient’s RNA. ASOs are highly targeted and very specific drugs. Typically ASOs “silence” their target, meaning that they decrease expression of the gene (such as SCN2A) and thus decrease unwanted activity. There are also some ASOs that have the potential to increase expression, or even correct the mutated gene. For neurological conditions, ASOs are typically administered intrathecally (via lumbar puncture) every few months. There are now multiple ASOs that have been approved by the FDA for various diseases.
2. Gene Therapy
Gene therapy refers to drugs that deliver genetic material to the patient. Typically these are one-time treatments, meaning patients are only treated once in their lifetime. This type of treatment can replace a dysfunctional gene that causes disease (such as SCN2A) with a healthy copy of the gene; inactivate or “knock out,” or “knock down” a mutated gene that is not working correctly; or, introduce a new gene into the body to help fight a disease.
3. Gene Editing
Gene editing is a form of therapy that often changes the patient’s genetic material (such as their DNA). This approach alters the genetic material of the patient by inserting, replacing, or deleting a DNA sequence. Some common types of gene editing that are being developed (not necessarily for SCN2A) include: CRISPR; Zinc Finger Nucleases (ZFNs); Transcription Activator-Like Effector Nucleases (TALEN); RNA editing.
Other therapeutic interventions
In addition to small molecules, ASOs, and gene therapy, there are other types of experimental treatments that may help. These include neuromodulators such as Vagus Nerve Stimulators (VNS), Responsive Neurostimulator (RNS), or Deep Brain Stimulators (DBS).
To learn more click here and view the video below:

Drugs in Preclinical Development
Before being tested on patients, a series of experiments are required to demonstrate that the prospective drug is both safe and effective. This stage of drug development is called preclinical (before the clinic/being used in patients). Preclinical experiments are often performed on cell lines in culture, small animal models, and also with computer simulations. Once a sufficient body of evidence is gathered (both efficacy and safety), the FDA may authorize an Investigational New Drug (IND) application which allows the medication to be used in clinical trials.
Drugs in Clinical Development
Clinical trials evaluate developmental medications and devices to determine whether they are both effective and safe. A series of trials are performed where patients are treated with the developmental drug or device and if these are successful the drug or device may become approved by regulators. Once approved, the drug or device becomes widely available for use by patients.
The National Organization for Rare Disorders (NORD) has a video series explaining the drug development process:
You can learn more about the drug approval process from the United States Food and Drug Administration.
Deciding to participate in a research study is a very personal decision, especially for an experimental treatment or clinical trial. It is especially complicated when we are asked to make decisions for children. Each of us has our own goals, desires, and individual risk tolerance for interventions, which may differ from those on your medical team. Even people in the same family may not share the same views about research.
First, figure out what your goals are for participating. Are those goals likely to line up well with the research project? For example, if you’re hoping that the treatment will “cure” a disorder but it is only designed to potentially help with temporary symptom management, it might not be a good fit.
Then, to help decide whether a research study is a good match for you, remember the acronym BRAIN:
Benefits - what good or positive outcome might come out of participating in this research? Sometimes there is the potential for a direct benefit to the participant, such as early access to an experimental treatment that might help. Sometimes the benefit is that you are contributing to general scientific knowledge, which could help with treatment discovery in the future. Sometimes it just feels good to help!
Risks - what harm or negative outcome might come out of participating in this research? These may be known or unknown side effects, some of which could be serious or life-threatening. Also ask yourself what harm or negative outcome might come out of NOT participating in this research? Is the risk of trying an experimental new treatment lower than the risk of the likely outcome if untreated? If you participate in this research, is there a risk that it will reduce or eliminate your options for future research opportunities?
Alternatives - what are your other choices or options? Your risk tolerance may vary quite a bit depending on whether you have other options, or if this research opportunity is your only choice at the time.
Intuition - what does your instinct or “gut feeling” tell you? Parents know their children best, and sometimes you just know in your heart that an intervention or research opportunity is or isn’t the right choice. It’s ok to trust that instinct, even if others don’t understand why you feel the way you do.
Numbers - is there any data or statistics to help you decide? How many people have already enrolled in the study? Perhaps an experimental study already has safety and tolerability data, which you can use to make an informed decision about possible risks and benefits. “N” can also stand for “No” or “Not Now” – participating in a clinical trial or other intervention is a big decision, and it’s ok to decline or defer your decision.
Know your rights as a research participant
You have many legal and ethical rights as a research participant, including:
- The right to be treated with respect and dignity
- The right to ask questions and to give informed consent in a language that you understand (see more below)
- The right to privacy and confidentiality of your data
- The right to decline to participate or to withdraw at any time
Most research centers will have a printed bill of rights or equivalent document available for research participants. Examples include this one from UCLA and this one from the NIH Clinical Center (research hospital).
Responsibilities
Being part of a research study can be a big commitment. While the researchers have many legal and ethical obligations toward the participant, it is also very important to understand participant responsibilities, too. Please try to adhere to the research protocol, and be honest with the research team if you are unable or unwilling to comply with the study requirements. Do not lie or exaggerate to fake eligibility to get into a clinical trial, or withhold information about side effects or symptoms before, during, or after participation. Inform all of your doctors and pharmacists of any research protocols you are doing to ensure that they make any necessary adjustments to your other medications or care plan.
What is informed consent?
Informed consent is not just a document that you sign. Informed consent is the process of communication that includes providing you with key information about a research study, and includes answering all of your questions. The research team provides an informed consent document that includes details about the study such as its purpose, how long it’s expected to last, tests or procedures that will be done as part of the research, specific participant requirements, and who to contact for further information. If you do not understand English, a translator or interpreter may be provided. The informed consent form explains the risks and potential benefits, and you are encouraged to ask lots of questions to make sure you understand. You can then decide whether to participate in the study, and will be asked to sign the consent form. The process of informed consent continues throughout the study, particularly if there are any changes.
To watch the FamilieSCN2A Town Hall conversation on Research Participation: Rights, Responsibilities, & Informed Consent, please visit https://youtu.be/S_EKFf1p2AE.
Here are some questions to help you with the informed consent process:
Where can I find out about clinical trials?
The website, www.ClinicalTrials.gov, provides a list of clinical trials in the United States and some other countries. There you can select a specific disorder and sign up for notification of new trials. In addition, a trial sponsor may advertise a trial through the medical community and patient advocacy groups.
Who is eligible to participate in a clinical trial?
Researchers provide a list of eligibility requirements so that people who meet the requirements may be enrolled in a trial. Each study is different so you may qualify for one trial but not another.
If I am eligible for a trial, how do I enroll?
If you think you meet the eligibility criteria for a clinical trial, contact the study organizers. The organizers will then set up a meeting and/or have you complete eligibility tests. If the team thinks you are an appropriate candidate who meets the requirements, they will help you enroll in the study.
What is informed consent?
Informed consent is the process of providing you with key information about a research study. If you do not understand English, a translator or interpreter may be provided. The research team provides an informed consent document that includes details about the study such as its purpose, how long it’s expected to last, tests or procedures that will be done as part of the research, specific participant requirements, and who to contact for further information. The informed consent document also explains the risks and potential benefits. You can then decide whether to participate in the study by signing the document. The process of informed consent continues throughout the study, particularly if there are any changes. Taking part in a clinical trial is voluntary and you can leave the study at any time.
What happens during a clinical trial?
Every trial is different and follows a defined protocol carefully designed to answer a specific medical question. Some studies involve observing behavior and taking surveys, while others require specimen collection, medical tests, and treatments. (Details are in the informed consent document.) All clinical trials gather participants’ personal information and monitor them closely throughout the entire study period.
Do trial medicines always work?
Fewer than 14 percent of all drugs in clinical trials receive approval from the FDA*. This means that the majority of investigational medications studied never make it to the consumer. When a new drug or medical device is studied for the first time in people, scientists don’t know exactly how the patients will react. They depend on study participants to help identify areas of success and improvement. (*January 2018 study from MIT Sloan School of Management in Biostatistics)
How long do clinical trials last?
Each clinical trial asks a specific question. Some questions will be answered more quickly than others which is why the length of each trial is different. Details for each particular study can be found in the informed consent document.
Where are trials held? Is there travel involved?
Most clinical trials take place where people already go for medical care–a doctor’s office or clinic. These locations may be near you or require travel. Some trials will have components that can be done at home with a visiting service provider. The details for each study can be found in the informed consent document.
Can I be in more than one clinical trial at a time?
There are no laws against participating in more than one clinical trial at the same time. However, you should check with the Primary Investigator of the current clinical trial you are participating in (or in each trial you are interested in) to see if that is an exclusion criteria.
Will trial sponsors pay for travel expenses?
Not all studies pay for travel to and from the trial site. When travel is included, payment takes many forms including reimbursement for gas or taxi services. Some sponsors will offer to provide a transportation service to the study location. Details related to travel costs will be in the informed consent document and you can ask about compensation for trial costs at any time. Please note that the IRS requires study payments of $600 or more to be reported on tax returns.
Will trial sponsors pay me to be in a clinical trial?
No rule or regulation requires sponsors to pay study participants; however, many offer compensation of some kind. Money, travel reimbursement, free health care, free screening exams and other tests are the most common forms of compensation. Details related to payment are in the informed consent document. Please note that the IRS requires study payments of $600 or more to be reported on tax returns.
What happens once a clinical trial is over?
Data collected from the trial determines if the drug, device, or treatment was successful. Studies that show success can move on to the next phase of clinical trials or on to the FDA for approval consideration.
Research Video Series
The FamilieSCN2A Foundation hosts regular community Town Hall meetings to help provide resources and education to the community. Check out the complete collection on YouTube.