Industry Relations Updates
Industry Relations Updates
American Epilepsy Society Annual Meeting, Chicago 12/2022
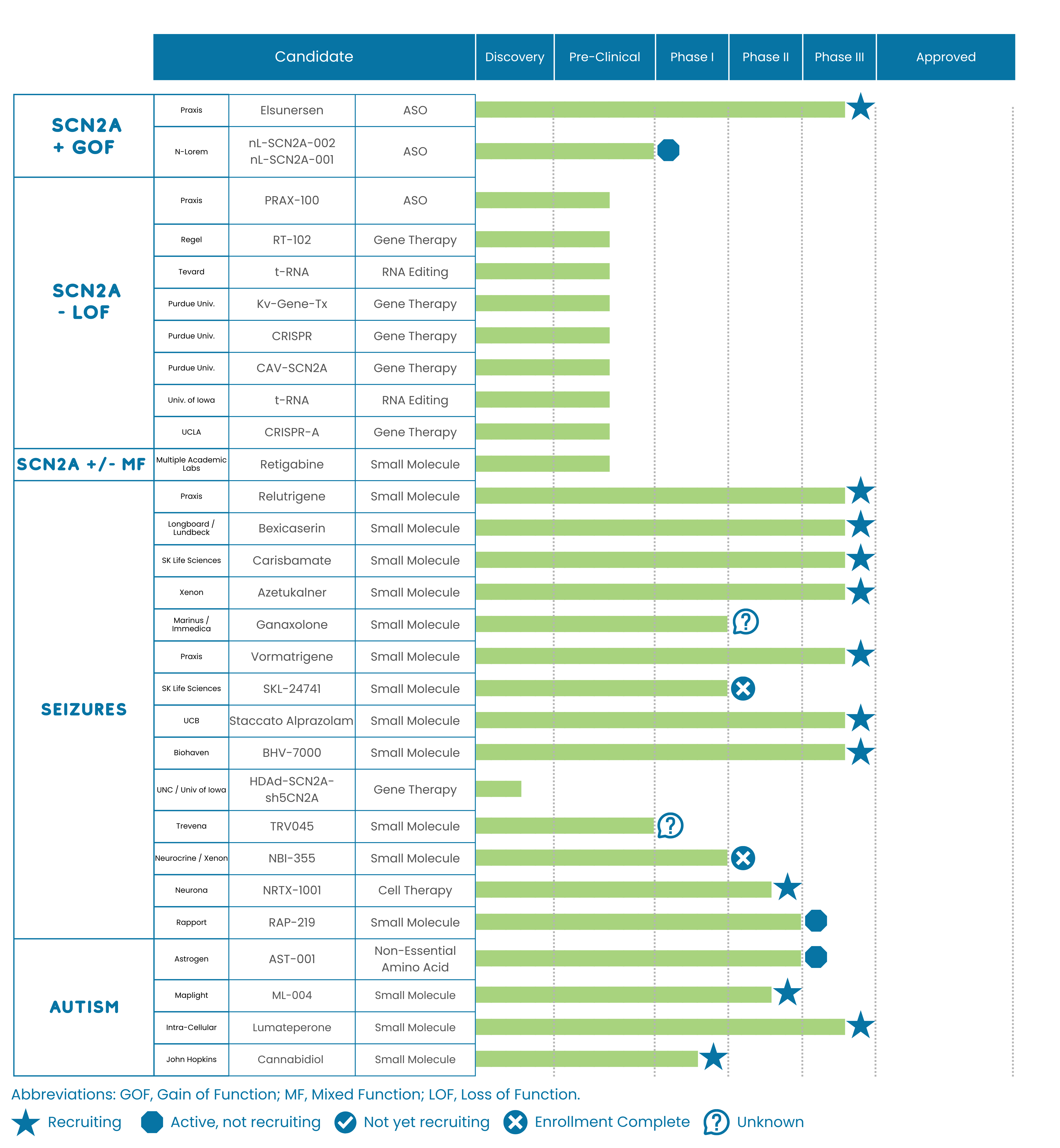
Longboard Pharmaceuticals plans to initiate a phase 1b/2a* clinical study for their drug LP352 sometime between January 1st and March 31 of 2022. This will be a basket study (enrolling many different Developmental and Epileptic Encephalopathies) which includes SCN2A. The full details of the inclusion/exclusion criteria have yet to be disclosed (we will share when available), but at this point we know:
1. The study will be for patients aged 18-65
2. For patients with Developmental and Epileptic Encephalopathies (several syndromes including SCN2A) with ≥ 4 motor seizures per month in the 3 months prior to screening and ≥ 4 motor seizures in the month of screening
3. Will exclude patients taking stiripentol, fenfluramine, or lorcaserin
4. This trial will be run in North America at ~25 different clinical sites.
It’s unclear at this point which SCN2A mutations could benefit most from this class of medication but this class of drug has proven to be beneficial in both Dravet Syndrome and LGS.
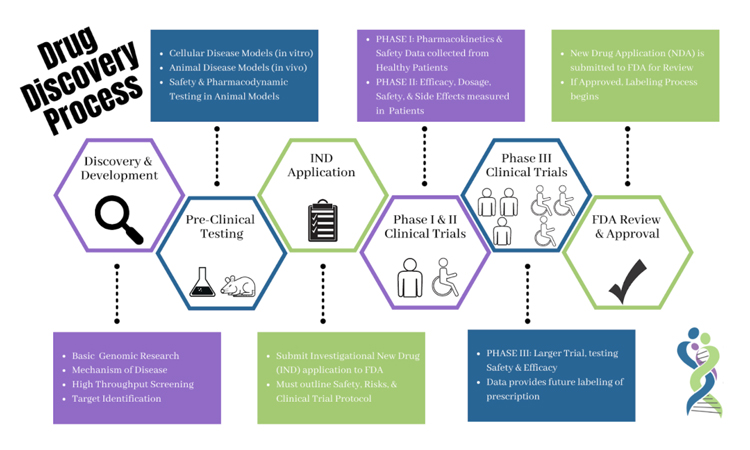
*Phase 1b/2a Trial means a human clinical trial conducted on study subjects with the disease or condition being studied for the principal purposes of gaining evidence of the safety and tolerability of, and potential pharmacological activity for, any product, as described in 21 C.F.R.
Praxis Precision Medicine reiterated their guidance to initiate a phase 2* clinical study in patients with DEEs (inclusive of SCN2A) for PRAX-562 in the first half of 2022 (between January 1 and June 30th). Typically with first half guidance this late in the year it often means that the trial has some potential to begin later than 1Q (otherwise the company would have refined their guidance to 1Q22). Details regarding eligibility for this study have yet to be disclosed but the compound is a persistent sodium channel blocker so the eligibility criteria is likely to be tailored towards patients with those mutations. We will share more details on the eligibility criteria of this study and trial locations when they become available. Although not discussed, the most recent guidance regarding PRAX-222 (their antisense oligo in preclinical development for SCN2A DEEs) is for a ph1/2 trial to also initiate in 1H22. Details regarding eligibility for this study have yet to be disclosed but the compound antisense oligo designed to reduce the expression of SCN2A so the eligibility criteria is likely to be tailored towards patients with gain of function mutations.
*In Phase 2 studies, researchers administer the drug to a larger group of patients (typically up to a few hundred) with the disease or condition for which the drug is being developed to initially assess its effectiveness and to further study its safety. A key focus of Phase 2 studies is determining the optimal dose or doses of a drug candidate, in order to determine how best to administer the drug to maximize possible benefits, while minimizing risks.
Additionally, our team formally met with four other biotech companies to discuss drug development in SCN2A. None of these companies currently have an active SCN2A program in IND*-enabling stages (meaning preclinical with a line of sight on moving to the clinic to treat patients). However our conversations were engaging and consistent with the types of conversations we have had with companies that are seriously considering initiating development on SCN2A. While none of these three companies are likely to offer an option for clinical trials for our community in the next year it was a clear positive to see this level of biotech interest in SCN2A. We view this as a strong signal that both science and technology are reaching a critical mass that could lead to something transformational for our kids.
*Investigational New Drug (IND) ApplicationLearn More
Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. The goal is to determine whether something is both safe and effective. Learn More