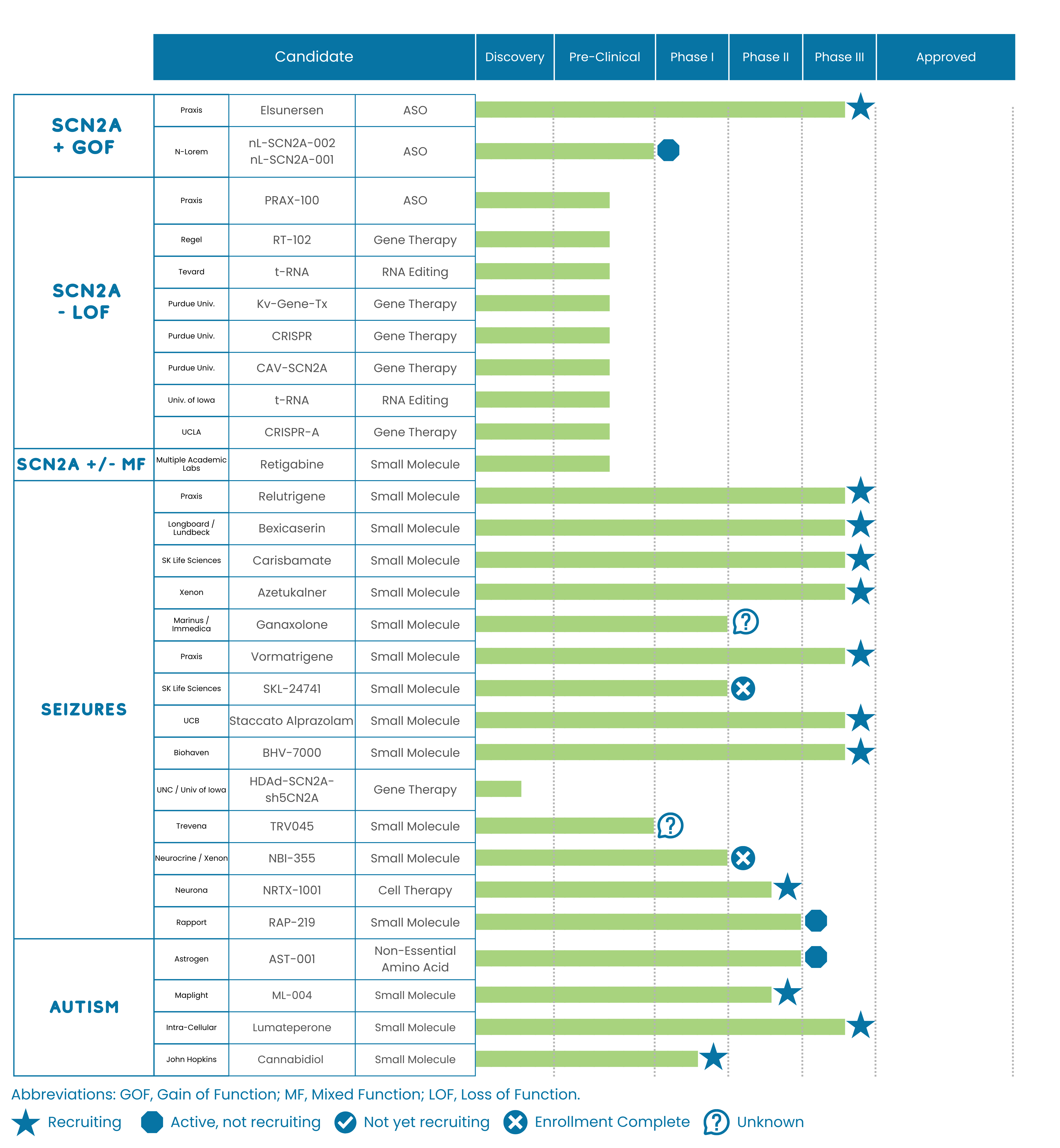
Promising Initial Relutrigine (Prax-562) Clinical Data
We will be hosting a SCN2A families only Town Hall this Thursday, 9/5/2024 at 12:00PM EST where we will be discussing these updates and data.
Data Implication to our SCN2A Community
The initial phase 2 data for Relutrigine (Prax-562) looks promising for our community, and the update that the trial is expanding to a registrational trial (instead of starting a new study) is good news because it could mean a shorter time to potential approval. Although it’s still early days with only seven SCN2A patients dosed, the initial data suggest that Relutrigine could have a material benefit for our early-seizure onset (more gain-of-function) SCN2A community. The trial showed improvements in seizures, communication, disruptive behaviors, and alertness—areas our community has highlighted as major unmet needs.
Topline Results of Phase 2 Relutrigine Study EMBOLD
Praxis Precision Medicines released initial results from the phase 2 EMBOLD study, which tested Relutrigine (PRAX-562) for the treatment of early-onset SCN2A and SCN8A developmental and epileptic encephalopathies (DEE). The trial demonstrated a 46% reduction in seizures compared to placebo, with a safety profile consistent with other drugs in this class. Patients and clinicians also noted early signs of benefit across various domains, including 1) disruptive behavior, 2) communication, 3) seizure severity and intensity, and 4) alertness. Furthermore, patients enrolled in the study’s long-term extension reported a 75% median reduction in seizures. Also reported that five patients experienced seizure-free periods longer than 28 days, with one patient exceeding 200 days without seizures.
About the EMBOLD Study
EMBOLD (cohort 1) was a randomized, placebo-controlled phase 2 study testing Relutrigine (Prax-562) in 16 patients (7-SCN2A and 9-SCN8A). Patients were treated for 4 months with daily relutrigine or a regimen of relutrigine daily for 3 months and matching placebo for 1 month, with the timing of placebo administration blinded to both participants and investigators. Key inclusion criteria included: documentation of severe DEE with mutation in SCN2A or SCN8A, ages 2-18 years of age, ≥ 8 countable motor seizures in 4 weeks preceding and during 28-day baseline observation, on stable anti-seizure medication doses for ≥1 month prior to screening.
About Relutrigine
Relutrigine (Prax-562) is an oral next-generation sodium channel blocker that is designed to be more selective to persistent sodium currents and thus have more selectivity to hyperexcitable sodium channels. It has an oral pediatric formulation and is dosed once daily in clinical studies.
Next Steps for the Program
Praxis is expanding the EMBOLD study into a registrational trial (a trial that may support approval by regulators like the FDA). Praxis plans to enroll 80 patients globally (combination of SCN2A and SCN8A patients) into this study.